A recent Journal of Experimental Medicine study reports that hybrid immunity induced by both coronavirus disease 2019 (COVID-19) vaccination and previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with persistent induction of virus-specific tissue-resident T-cells in the nasal cavity that are vital for early-phase protection against future SARS-CoV-2 infection.
Study: SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8+ and CD4+ T cells of broad specificity. Image Credit: Kateryna Kon / Shutterstock.com
Background
The human upper respiratory tract is the primary site of SARS-CoV-2 entry and replication due to the high expression of the angiotensin-converting enzyme 2 (ACE2) receptor in nasal epithelial cells. Nasal resident T-cells that rapidly recognize SARS-CoV-2-infected cells have a crucial role in eliminating the virus at the early phase of infection.
According to available literature, SARS-CoV-2-specific tissue-resident T-cells can be detected in the upper and lower respiratory tracts of infected or healthy individuals. However, the magnitude of the T-cell response induced by vaccination, infection, or both are not fully understood.
In the current study, scientists evaluate the presence, functionality, and persistence of SARS-CoV-2-specific nasal-resident T-cells in vaccinated individuals with or without breakthrough infection.
Characterization of nasal-resident T-cells
T-cells collected from the nasal mucosa of participants were subjected to phenotypic analysis and different T-cell assays. No significant differences in the phenotype and frequency of nasal T-cells were observed between vaccinated participants with and without breakthrough infection.
Regarding viral specificity, SARS-CoV-2-specific nasal-resident CD4+ and CD8+ T-cells were detected almost exclusively in vaccinated participants with breakthrough infection. Conversely, none of the vaccine recipients without breakthrough infection exhibited virus-specific nasal-resident T-cells, despite the presence of spike-specific T-cells in the blood. This finding indicates that natural infection is needed to induce virus-specific T-cell immunity in the nasal cavity.
In vaccinated participants with breakthrough infection, nasal T-cells were found to target various structural and non-structural proteins of SARS-CoV-2, in addition to targeting the viral spike protein. This type of T-cell response is particularly vital against immune escaping SARS-CoV-2 variants like Omicron, as non-structural protein-specific T-cells are less likely to be affected by mutations commonly observed in the spike protein.
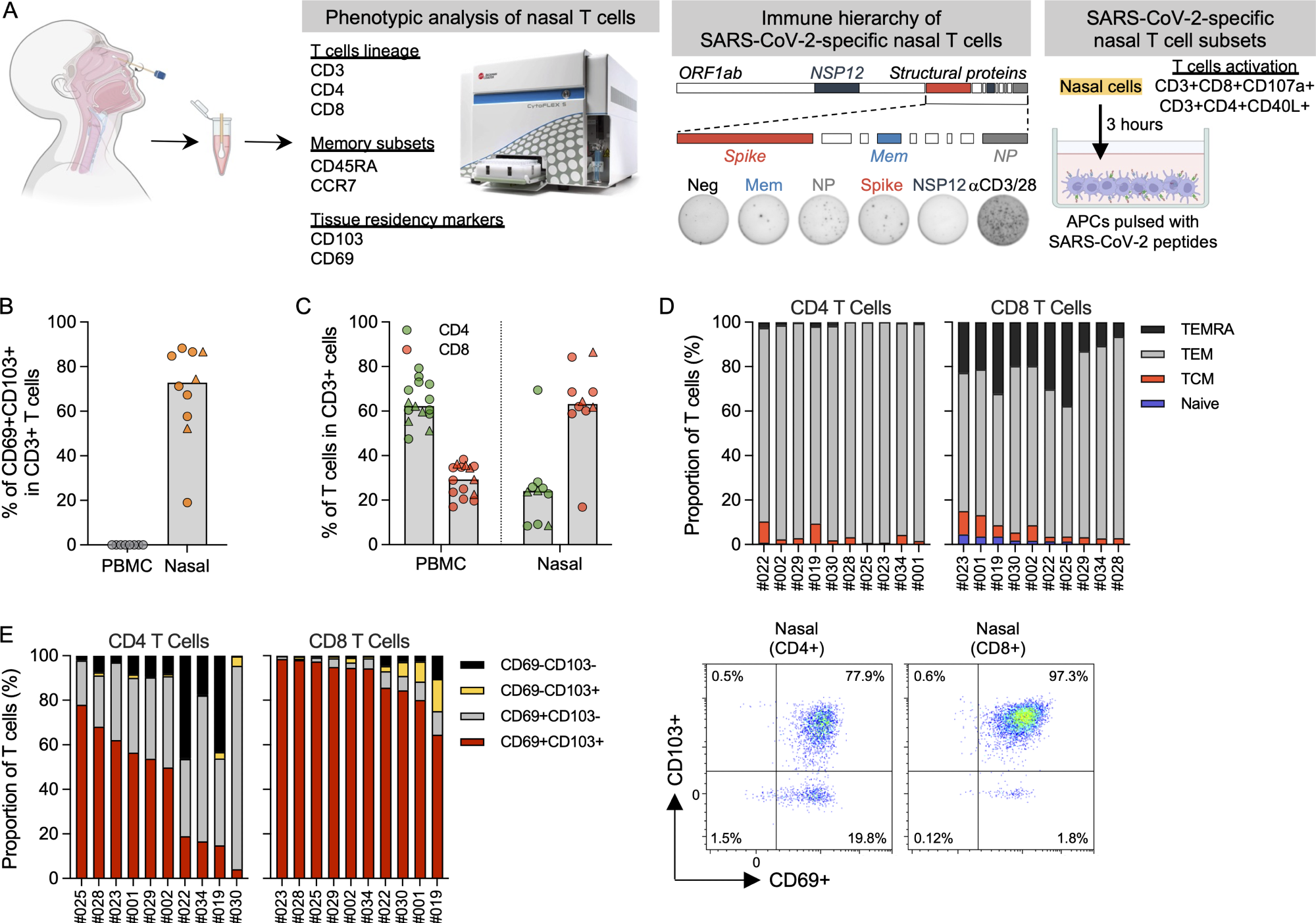 Phenotypic analysis of T cells in nasal secretion. (A) Schematic of experimental design. (B) Frequency of tissue-resident T cells present in PBMCs (n = 8) and nasal secretion (n = 10). Convalescent vaccinees are indicated by a triangle symbol. (C) Frequency of CD4 and CD8 T cells present in PBMCs (n = 14) or nasal cells (n = 10). Convalescent vaccinees are indicated by a triangle. (D) Proportion of naive (CCR7+CD45RA+), central (CCR7+CD45RA−; TCM), effector (CCR7−CD45RA−; TEM) and terminally differentiated (CCR7−CD45RA+; TEMRA) memory CD4+ and CD8+ nasal T cells (n = 10). (E) Proportion of tissue-resident marker (CD69 and CD103) expression on CD8 and CD4 nasal T cells (n = 10) and corresponding representative plots.
Phenotypic analysis of T cells in nasal secretion. (A) Schematic of experimental design. (B) Frequency of tissue-resident T cells present in PBMCs (n = 8) and nasal secretion (n = 10). Convalescent vaccinees are indicated by a triangle symbol. (C) Frequency of CD4 and CD8 T cells present in PBMCs (n = 14) or nasal cells (n = 10). Convalescent vaccinees are indicated by a triangle. (D) Proportion of naive (CCR7+CD45RA+), central (CCR7+CD45RA−; TCM), effector (CCR7−CD45RA−; TEM) and terminally differentiated (CCR7−CD45RA+; TEMRA) memory CD4+ and CD8+ nasal T cells (n = 10). (E) Proportion of tissue-resident marker (CD69 and CD103) expression on CD8 and CD4 nasal T cells (n = 10) and corresponding representative plots.
Systemic and nasal mucosal T-cells
The analysis of virus-specific systemic and nasal mucosal T-cells in participants with breakthrough infection revealed that the proportion of spike-specific T-cells is higher in the blood than in the nasal mucosa.
In contrast, a predominant presence of non-structural protein-specific T-cells was observed in the nasal mucosa of these participants. These findings indicate that SARS-CoV-2 infection induces broad-spectrum virus-specific T-cell responses in the nasal mucosa that are not dominated by the vaccine-induced spike-specific T-cell response in the blood.
Regarding the functionality of T-cells, nasal and systemic T-cells were found to exhibit differential patterns of cytokine secretion. In vaccinated participants with breakthrough infection, systemic T-cells showed a broader cytokine secretion profile than nasal T-cells. However, in vaccinated participants without breakthrough infection, no cytokine secretion was observed for nasal T-cells.
Persistence of SARS-CoV-2-specific nasal-resident T-cells
Nasal samples collected from vaccinated participants with breakthrough infection at different timepoints following recovery from infection were analyzed to understand the persistence of virus-specific T-cells. To this end, nasal-resident T-cells persist for more than 140 days, with the potential waning of T-cell responses observed three to four months after recovery from infection.
Study significance
SARS-CoV-2 breakthrough infection induces tissue-resident T-cells in the upper respiratory tract that are specific for a wide range of SARS-CoV-2 proteins. These multi-specific nasal T-cells persist for more than 140 days following recovery from COVID-19.
However, COVID-19 vaccinated individuals do not display similar T-cell responses in the upper respiratory tract. Despite inducing detectable levels of spike-specific T-cells in the blood, COVID-19 vaccination fails to trigger a virus-specific nasal-resident T-cell response.
Overall, the current study highlights the immunological signatures of hybrid immunity against SARS-CoV-2.








 User Center
User Center My Training Class
My Training Class Feedback
Feedback












Comments
Something to say?
Log in or Sign up for free