Breast cancer is the most common cancer in women and accounts for the most cancer-related deaths among women globally. It is a heterogeneous malignancy that is classified into different subtypes based on the status the oestrogen receptor (ER) and progesterone receptor (PR) — collectively known as hormone receptors (HRs) — and the human epidermal growth factor receptor 2 (HER2, also known as ERBB2). HR and HER2 status determine drug treatment options.
Current treatments
HER2-positive breast cancer. Approximately 20% of breast cancers are HER2-positive. Trastuzumab (Herceptin, Roche) was the first HER2-targeting agent to be approved (in 1998). Since then, a plethora of HER2-targeting agents have been approved, including pertuzumab (Perjeta, Roche), trastuzumab emtansine (T-DM1; Kadcyla, Roche), lapatinib (Tykerb/Tyverb, Novartis), neratinib (Nerlynx, Puma), trastuzumab deruxtecan (Enhertu, Daiichi Sankyo/AstraZeneca), tucatinib (Tukysa, Seagen/Pfizer) and margetuximab (Margenza, MacroGenics).
Trastuzumab plus chemotherapy with or without pertuzumab is the adjuvant or neoadjuvant standard of care for early-stage disease. Trastuzumab emtansine and neratinib are adjuvant treatment options. Pertuzumab plus trastuzumab and chemotherapy is the first-line and trastuzumab emtansine is the second-line standard of care for metastatic disease. However, trastuzumab deruxtecan (approved in 2019), tucatinib plus trastuzumab and capecitabine, neratinib plus capecitabine, and margetuximab plus chemotherapy (all approved in 2020) are beginning to erode trastuzumab emtansine and trastuzumab prescriptions for recurrent disease. Trastuzumab deruxetecan is also being tested as a post-neoadjuvant treatment, and tucatinib in combination with trastuzumab emtansine is being tested for patients who are trastuzumab-treated and taxane-treated with advanced or metastatic disease.
HR-positive/HER2-negative breast cancer. Early-stage disease is treated with hormonal therapy, typically for 5–10 years. Patients with intermediate and high-risk disease may also receive chemotherapy prior to hormonal treatment. The first-line standard of care for metastatic disease is a cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor — palbociclib (Ibrance, Pfizer), ribociclib (Kisqali, Novartis) or abemaciclib (Verzenio/Verzenios/Virginio, Eli Lilly) — in combination with endocrine therapy. These therapies gained regulatory approval in 2015–2017 and have received successive label expansions; they are also being assessed in late-phase trials as adjuvant treatments for early-stage disease. The pivotal monarchE trial, assessing adjuvant abemaciclib, has met its primary end point of invasive disease-free survival.
In 2019, the FDA approved the PI3K inhibitor alpelisib (Piqray, Novartis) for PIK3CA-mutated (approximately 40% of patients) advanced or metastatic disease following progression with an endocrine therapy. Everolimus (Afinitor, Novartis) with exemestane is also a treatment option for HR-positive/HER2-negative recurrent disease.
Triple-negative breast cancer. In 2019, the PDL1 inhibitor atezolizumab (Tecentriq, Roche) was granted FDA accelerated approval for PDL1-positive (approximately 40% of patients) advanced or metastatic disease, in combination with nanoparticle paclitaxel (Abraxane, Celgene). The approval of atezolizumab was based on progression-free survival data from a phase III trial (IMpassion130); however, in August 2020, atezolizumab (plus paclitaxel) failed to meet progression-free survival as a co-primary end point in another trial (IMpassion131), which treated the same population as in IMpassion130. Other phase III trials of atezolizumab are in early-stage triple-negative and HER2-positive disease.
The PD1 inhibitor pembrolizumab (Keytruda, Merck & Co.) in combination with chemotherapy was approved by the FDA in November 2020 for the treatment of patients with locally recurrent unresectable or metastatic triple-negative breast cancer who express PDL1. Approval was based on the KEYNOTE-355 trial. A supplemental Biologics License Application is under FDA review for neoadjuvant pembrolizumab in combination with chemotherapy followed by adjuvant pembrolizumab for early-stage disease. The prescription drug user fee act (PDUFA) date is March 29, 2021. Pembrolizumab is also being assessed in early-stage ER-positive/HER2-negative disease.
Two poly-ADP ribose polymerase (PARP) inhibitors are approved for chemotherapy-pretreated, germline BRCA1/2-mutated, HER2-negative advanced or metastatic breast cancer. Prevalence of BRCA1/2 mutations in triple-negative (15%) and HR-positive/HER2-negative (3–4%) breast cancer is low. Olaparib (Lynparza, AstraZeneca) was first to market, followed by talazoparib (Talzenna, Pfizer) (both FDA approved in 2018); Olaparib is also being assessed as an adjuvant treatment for high-risk BRCA1/2-mutated HER2-negative disease.
Sacituzumab govitecan (Trodelvy, Immunomedics) is a trophoblast cell surface antigen 2 (TROP2)-targeted antibody–drug-conjugate (ADC). It gained FDA accelerated approval in 2020 for third-line and later-line metastatic triple-negative disease based on a single-arm phase II trial. A confirmatory phase III (ASCENT) trial is ongoing. It is also in phase III trials for pretreated advanced or metastatic HR-positive/HER2-negative disease (TROPICS-02 trial), and for early-stage HER2-negative disease regardless of HR status (SASCIA trial).
Emerging therapies
The breast cancer late-phase pipeline is diverse, spanning approved drug classes and novel mechanisms of action (including inhibitors of AKT and CXCR4) in a variety of treatment settings (Table 1).
Table 1 | Select therapies in the late-phase pipeline for breast cancer
Product |
Companies |
Target or MOA |
Development status |
Oral paclitaxel + encequidar (Oraxol) |
Athenex |
Taxane |
Preregistration |
Trastuzumab duocarmazine |
Byondis |
HER2 |
Phase III |
Nivolumab (Opdivo) |
Bristol Myers Squibb/Ono |
PD1 |
Phase III |
Ipatasertib |
Roche/Genentech/Chugai |
AKT |
Phase III |
Capivasertib |
AstraZeneca |
AKT |
Phase III |
Balixafortide |
Polyphor |
CXCR4 |
Phase III |
Veliparib |
AbbVie |
PARP |
Phase III |
Elacestrant |
Radius Health |
SERD |
Phase III |
Tesetaxel |
Odonate Therapeutics |
Taxane |
Phase III |
Adagloxad simolenin |
OBI Pharma |
Vaccine |
Phase III |
GDC-0077 |
Roche/Genentech |
PI3K |
Phase II/III |
Lasofoxifene |
Sermonix Pharmaceuticals |
SERM |
Phase II |
Trastuzumab duocarmazine (Byondis) is a HER2-targeting ADC that is being assessed in pretreated advanced or metastatic HER2-positive breast cancer (TULIP trial), the same population that multiple other HER2-targeting agents are approved for. PD1 inhibitor nivolumab (Opdivo, Bristol Myers Squibb/Ono) is in development for early-stage, previously untreated, high-risk ER-positive/HER-negative disease (CheckMate 7FL trial), mirroring an ongoing phase III trial (KEYNOTE-756) for pembrolizumab.
Two AKT inhibitors are in development for advanced and metastatic breast cancer. One of these, ipatasertib (Roche) plus chemotherapy is being evaluated with (IPATunity170) or without (IPATunity130) atezolizumab as a first-line treatment for triple-negative disease. However, primary results from the IPATunity130 trial failed to demonstrate a clinical benefit for ipatasertib in combination with paclitaxel. Ipatasertib is also being assessed in combination with palbociclib for HR-positive/HER2-negative disease (IPATunity150). The other, capivasertib (AstraZeneca) is being assessed for triple-negative (CAPItello290) and HR-positive/HER2-negative (CAPItello291) disease.
Balixafortide (Polyphor) is a CXCR4 inhibitor. It is being combined with eribulin (Halaven, Eisai) for pretreated HER2-negative recurrent or metastatic breast cancer. Veliparib (AbbVie) is also being developed for patients who are HER2-negative, but for BRCA1/2-mutated tumours; unlike approved PARP inhibitors, veliparib is being evaluated with chemotherapy. Two hormonal therapies are in pivotal trials for pretreated ER-positive/HER2-negative metastatic breast cancer: elacestrant (Radius Health) and lasofoxifene (Sermonix Pharmaceuticals). Other drugs in the late-phase pipeline include two oral taxanes, a PI3K inhibitor and a vaccine targeted to Globo H (Table 1).
Market indicators
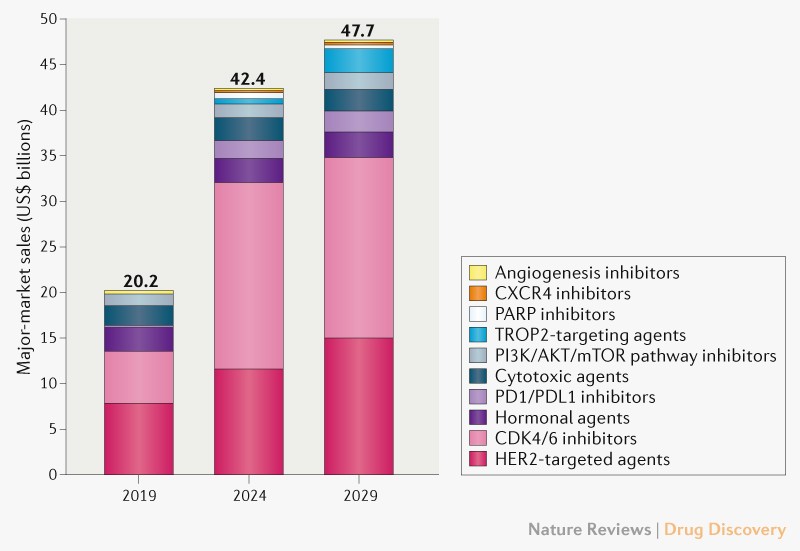
In 2019, the breast cancer market totalled US$20.2 billion and was dominated by sales of therapies targeting HER2 or CDK4/6 (68% of sales). Despite competition from generic and biosimilar agents during our 2019–2029 forecast period, including competitors for the key agents trastuzumab and palbociclib, the breast cancer market is forecast to grow 9% annually to $47.7 billion (Fig. 1). The continued uptake and expanded use of CDK4/6 inhibitors and entry of 14 premium-priced agents, including therapies approved in 2019 or 2020 (trastuzumab deruxtecan, tucatinib, margetuximab, atezolizumab, pembrolizumab, alpelisib and sacituzumab govitecan) will fuel this sales growth.
By 2029, the CDK4/6 and HER2-targeted agents are expected to maintain their share (73%) of breast cancer sales, attributing for approximately $20 billion and $15 billion, respectively. Trastuzumab deruxtecan is expected to become the top-selling HER2-targeted drug owing to its anticipated broad use for HER2-positive, HR-positive/HER2-negative and triple-negative cancers and its long treatment duration. The anticipated label expansion of two CDK4/6 inhibitors (abemaciclib and ribociclib) for early-stage HR-positive/HER2-negative breast cancer will also drive sales, adding $14 billion in 2029 (69% of drug class sales) in our forecast, with abemaciclib contributing $13 billion (94%) of adjuvant sales.
New drug classes are expected to enter the breast cancer market, diversifying treatment options for patients who are triple-negative and HR-positive/HER2-negative. However, sales of some therapies will be constrained by their biomarker-defined populations, approvals in smaller late-line populations, as well as strong competition from established and emerging therapies.








 User Center
User Center My Training Class
My Training Class Feedback
Feedback












Comments
Something to say?
Log in or Sign up for free